[Policy Recommendations] Furthering the Development of Precision Cancer Medicine —Proposals for Effective Policy Changes Based on Key Characteristics of Precision Medicine in Cancer Treatment— (September 20, 2022)
Health and Global Policy Institute (HGPI) has released a policy Recommendations “Furthering the Development of Precision Cancer Medicine—Proposals for Effective Policy Changes Based on Key Characteristics of Precision Medicine in Cancer Treatment—”
Executive Summary
- In particular, “precision medicine” is high expected to play a critical role in cancer treatment in the future. This field, sometimes referred to as precision cancer medicine, is a form of treatment tailored to individuals based on the genetic mutations and other characteristics of their cancer.
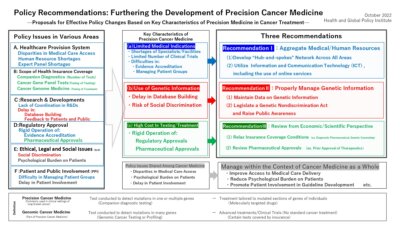
- To further develop precision cancer medicine, it will be necessary to overcome policy issues in various areas including: (i) access to medical care, (ii) human resource development, (iii) research and development, (iv) regulatory approval and health insurance coverage, and (v) patient support.
- Although many of these issues are shared among various forms of cancer medicine, precision cancer medicine has the following three key characteristics which further complicate efforts to address them, namely:
(a) the number of medical indications for its use are still quite limited
(b) it involves the use of genetic information
(c) the costs of testing and treatment are often expensive
As such, attempts to address the policy issues mentioned above will be more effective if they have a firm basis in these key characteristics.
- Based on a recognition of this issue, Health and Global Policy Institute (HGPI) offers the following recommendations to promote the development of precision cancer medicine
Recommendation I: To effectively allocate human resources and aggregate knowledge, a “hub-and spokes” network should be developed across all areas, including (i) healthcare delivery systems, (ii) human resources, (iii) research and clinical trials, and (iv) patient support measures. When doing so, proactive steps to adopt information and communication technology (ICT), including the use of online services, should be taken to streamline the aggregation of information and medical resources.
Recommendation II: While establishing data repositories for genetic information, legislation prohibiting discrimination based on genetic information should be enacted and public awareness activities should be conducted.
Recommendation III: Regulatory approval, health insurance coverage, and other conditions governing the use of precision cancer medicine should be revised to be made more scientific and rational in a manner that complements the key characteristics of precision cancer medicine and practical needs in clinical settings.
HGPI kicked off an initiative called the “Project for Considering the Future of Precision Medicine with Industry, Government, Academia, and Civil Society” in FY2021. The recommendations offered in this document are based on repeated discussions we have held with various experts in this area as part of that initiative. HGPI strongly hopes these recommendations will be utilized in future cancer control measures to further develop patient-centered healthcare.

Advisory board (honorific titles omitted; in Japanese syllabary order):
- Reiko Akizuki (Director, Oncology Department, Medical Affairs Division, Janssen Pharmaceutical K.K.)
Kosuke Iijima (Head, Foundation Medicine Business Department, Chugai Pharmaceutical Co., Ltd.)
Ataru Igarashi (Associate Professor, Unit of Public health and Preventive Medicine, Yokohama City University School of Medicine)
Mitsuho Imai (Project Assistant Professor, Shinanomachi Cancer Center, School of Medicine, Keio University)
Hiroji Iwata (Vice Director and Chief, Department of Breast Oncology, Aichi Cancer Center Hospital)
Sotaro Enatsu (General Manager, Oncology Business Unit, Research and Development and Medical Affairs, Eli Lilly Japan K.K.)
Atsushi Otsu (Director, National Cancer Center Hospital East)
Tomohiro Kuroda (Professor, Division of Medical Information Technology and Administration Planning, Kyoto University Hospital)
Shinji Kosugi (Professor, Medical Ethics and Medical Genetics, Department of Social Medicine, Graduate School of Medicine and Faculty of Medicine, Kyoto University)
Naomi Sakurai (President, Cancer Solutions Co., Ltd)
Chizuko Sakashita (Project Assistant Professor, Hematology, Advanced Therapeutic Sciences, Medical and Dental Sciences, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University)
Kuniko Sunami (Department of Laboratory Medicine, National Cancer Center Hospital)
Junpei Soeda (Head, Japan Medical Affairs, Japan Oncology Business Unit, Takeda Pharmaceutical Company Limited)
Yusuke Tsugawa (Associate Professor, David Geffen School of Medicine (Internal Medicine), University of California, Los Angeles (UCLA); Associate Professor, Health Policy and November 2022 Health and Global Policy Institute 30 Management; Faculty Associate, UCLA Center For Health Policy)
Mamoru Narukawa (Professor, Department of Clinical Medicine (Pharmaceutical Medicine), Kitasato University School of Pharmacy)
Kazuo Hasegawa (Representative, NPO Lung Cancer Patient Network One Step)
Yoshiyuki Majima (Chairman, NPO PanCAN Japan)
Tetsuya Mitsudomi (Professor/Senior Staff, Division of Thoracic Surgery, School of Medicine, Kindai University)
Kaori Muto (Professor, Department of Public Policy, The Institute of Medical Science, The University of Tokyo)
Takayuki Yoshino (Director, Department of Gastrointestinal Oncology, National Cancer Center Hospital East)
Sponsors (in Japanese syllabary order):
- Takeda Pharmaceutical Company Limited.
Chugai Pharmaceutical Co., Ltd.
Eli Lilly Japan K.K.
Janssen Pharmaceutical K.K.
■関連する項目
[Event Report] The First Advisory Board Meeting of the Project for Considering the Future of Precision Medicine with Industry, Government, Academia, and Civil Society (December 14, 2021)
[Event Report] The Second Advisory Board Meeting of the Project for Considering the Future of Precision Medicine with Industry, Government, Academia, and Civil Society (February 22, 2022)


